
As Apple looks about for ways to extend the iPhone-iPad ecosystem, personal health care seems like a natural. The iOS devices are already extremely popular with health care professionals, and apps that supply health information to doctors are among the most expensive products in the App Store. In a Tech.pinions Insider article, John Kirk figures that health care-based wearables could be the next big frontier for Apple. While I agree that health care will grow as a important element of the iOS ecosystem, Apple will likely leave the devices to third parties that are better equipped to compete in this specialized market.
The reason, in a word, is regulation. Throughout its history, Apple has wisely steered clear of highly regulated markets, whose hallmark is slow moving cautiousness. And markets don’t get much more regulated, or slower moving, than health care. Anything the Food & Drug Administration classifies as a Class II medical device ((Class I devices, “not intended to help support or sustain life or be substantially important in preventing impairment to human health” (Code of Federal Regulations) are simple items such as canes or walkers and can generally sold without advance approval as long as they are safe. Class II devices, which includes most things that do any measurement or assessment, require proof of both safety and effectiveness. Class III devices are the most regulated and are almost never sold except through medical professionals.)) , especially if there is anything novel about it.
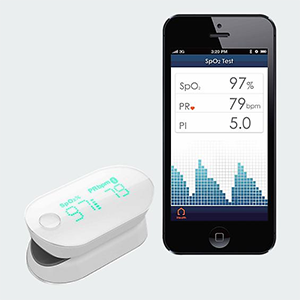
The 23 and Me Challenge. The same sort of regulatory regime that cracked down on the Google-backed genetics company 23 and Me can make the consumer market for medical devices a big challenge. The AliveCor Heart Monitor (photo above) is a two-contact electrocardiogram that works as a case for an iPhone or a Samsung Galaxy S3 or S 4. But even if you know how to read an EKG yourself, you cannot just use it that way (at least not legally.) Although you can now order the $199 device without a prescription, you must send the readings off for interpretation by either an AliceCore “cardiac technician” or clinical review by a board-certified cardiologist.
An executive of one company, who wanted to remain anonymous because of ongoing negotiations with the FDA, gave me an example of the frustrations. As part of a comprehensive home care system, the company had developed a connected blood glucose monitor. A diabetic patient would take regular readings, which would both be sent to a physician and measured against a database to give an interpretation and advice, say to go for a walk or eat something soon to regulate the glucose level. The FDA was fine with the physician notification part, but said absolutely no to advice and interpretation because that was practicing medicine and could only be done by a licensed professional.
The Challenge of Integration. Another impediment to entry into this market that the for mobile healthcare devices–glucose meters, pulse oximeters, blood pressure monitors, or pretty much anything else–they need to be part of an integrated system that pumps data into providers’ systems and specifically into their electronic medical records. This is an extremely complicated integration task and is almost certainly left to companies such as Verizon, AT&T, and Qualcomm that have made major investment in these systems. And even then it isn’t easy. It took Verizon more than two years to win full FDA approval for its machine-to-machine Healthcare Enabled solutions network.
The complexity of the approvals process makes for a painfully slow path to market. The realities of the system means that most purchasing is done not by consumers and often not healthcare providers, but third-party payers. Prices are high and markets are relatively small.
Just the sort of business the Apple hates. I do expect that Apple will enter the wellness and fitness market with sensor devices that take advantage of the M7 sensor chip, which made its debut in the iPhone 5s.This is a crowded field, with companies such as Fitbit, Jawbone, and Nike already established as incumbents, but I would be surprised to see Apple do something new and exciting in coming months. But the broader healthcare business is a cup Apple may well prefer to let pass its lips.

I think health-related uses of wearable accessories will be natural, but I see Apple’s strategy more as sensors/accessories that gather data and can interact with other things. Imagine a gorgeous bracelet packed with sensors that can not only monitor a bunch of health info all day long (and send it to your iPhone to be crunched and charted), but that same sensor bracelet can take on other functions, perhaps some wallet action, some portion of ID, a motion controller for a beefed up Apple TV game box, a trigger for various ‘smart home’ jobs, there’s so many possibilities. Apple builds computers, so I would think of accessories like this as devices that can take over certain computing jobs. And for now these devices will need to be tied to your iPhone. As the iMac was the hub for your digital life, the iPhone is the hub for your mobile life.
The best way to fight the archaic regulations is to introduce the devices abroad. Once they become established outside, people here would look like fools and conservative. A few years ago, on line signatures were not legal. Now one can do every transaction through PDFs and software that make things easy. It is only a matter of time. May be Apple and other companies can introduce these devices in countries like China, India etc in the name of leap frogging over “Western” countries. That would heat things up and no one in the West wants those countries to be ahead in any way. Here is a “divide and rule” method that can work 🙂
Overseas medicine also gave us Thalidomide and other drugs that caused serious problems. FDA foot dragging prevented or reduced those problems in the US.
It is vice versa with not removed. Apple is the future of healthcare. The momentum of Apple’s entry will bring healthcare mainstream for many. There are many devices available from many but none of them have able to make their devices mainstream.
Never say never. Apple knows that how to move into complex systems in small steps. Cf. what the iPhone did in enterprise: suddenly nontech managers insisted on tech folks patching them in. This, after twenty years of groveling to IT.
The question is not whether Apple, with enough effort, could prevail in this market. They seem to be good at whatever they try hard enough. The question is whether Apple wants to, or should want to. It doesn;t seem like their game.
Apple was Apple to work its way through a clever campaign of subverting IT managers. (They don;t get nearly enough credit for the iOS conquest of the enterprise being a deliberate strategy, but that’s another story.) The problem is you cannot subvert the federal government and especially the FDA. They’re smart, they’re tough, they have a distinct ideological outlook that transcends politics–the precautionary principle–and they have the resources to outlast anyone. Ask 23 and Me about the chances of succeeding at an end run.
All Apple needs to provide are the health documentation tools. That it can do. (Without them wealth rules health.) We will do the rest.
Apple doesn’t subvert; it simply offers smart tools to replace dumb ones.
Mr. Wildstrom is 100% correct. FDA is, first and foremost, a law enforcement agency. Make no mistake about it, if these devices are marketed and sold with any expectation of accuracy, and not as “mood rings”, then these are medical devices. In enforcing the law, FDA looks at EVERYTHING. From the components to the process, from the HW to the SW, to the color Mr. Cook may choose to paint the machines that do the assembly. (If you choose to change the paint, lather-rinse-repeat). Apple is absolutely not amenable to such oversight. They have problems with a court appointed monitor for chrissakes!
Bottom line, it’s doable, but not by today’s Apple.
Initially the iWatch won’t need FDA approval because it is still secret and Apple wouldn’t want to let them see it before it is released and of course the delays involved for approval. Once it is released then they can add more features and capabilities to it that do require FDA approval and add them once they are approved. Sure it takes a long time, but Apple has the cash and the resources and any competitor will have to do the same to compete. In fact Apple could take a short cut and acquire tech that has passed the FDA review process and integrate it. For instance they could purchase Alivecor and integrate that tech into every iPhone or perhaps the iWatch. No other smart phone company could come close to duplicating it because of the FDA hurdles involved. With Apple’s economies of scale it probably should add too much cost to the product. I don’t know if I need an EKG in my watch or phone but it would still be very cool.
I think the real break through lies when the apps can make recommendations. It can’t be rocket science to to have an app tell someone to eat some fruit when their blood glucose levels drop. Also I would imagine that the computational abilities of todays smartphones are sufficient to read an EKG. I don’t think it would really be that hard to program. The FDA just has to catch up to the present.
I am looking forward to my own personal Tricorder, perhaps in the iPhone 12
With the exception of klahanas, who sounds like he has had dealings with the FDA, there seems to be almost a willfulness on the part of commenters to ignore the challenges posed by federal regulation. Just talk to one of the companies that has done a Bluetooth health sensor (not just a glorified pedometer) and they will tell you that getting these things through approval is tough. And it’s not just the device–it’s the software that processes the data, the networks that transmit the data, the interfaces that connect the data to an EMR. And getting it to market outside the U.S. will do you know good whatsoever because the FDA prides itself on starting the approval process de novo.
It all goes back to the 1960s and thalidomide. The FDA still sees as its finest moment the day Dr. Frances Kelsey kept thalidomide, which had won widespread regulatory approval in Europe but which turned out to cause terrible birth defects when taken by pregnant women, off the U.S. market. Ever since then, the FDA has, for better or worse, practiced an extreme form of the precautionary principle: It’s responsibility, above all, is to prevent harm. The agency recently had to be pushed hard to grant emergency approval (to stop an epidemic) of a meningitis vaccine that was widely approved in Europe.
I can’t imagine a a surer formula for unpleasantness than the FDA’s steadfastness meeting Silicon Valley’s “move fast and break things” attitude. But I know that for the foreseeable future, I’m betting on the FDA.
I think the question is more along the lines of how far Apple can tread before the regulatory issues become onerous. For example, what kind of regulation/approval does a bathroom scale need to go through? Surely there’s a difference between gathering data and the extra step of recommending a course of action based on that data (which should involve a medical professional). But perhaps not?
That’s an interesting example. The scale, even a wi-Fi or Bluetooth scale, is a Class I device and would not require approval—as long as it just talks to your phone or even a cloud database that makes no medical claims. Once it becomes part of a system that reports your weight to your doctor (and such scales and systems exist; they are especially useful in the monitoring of congestive heart failure, where an unexplained weight gain is an important symptom of fluid retention that can be dangerous) then it moves into Class II regulated territory. The important thing is you have to know just where the lines are and be careful to stay on the right side of them. It’s not really that hard, but a lot of tech firms have such contempt for regulation that they fail to be careful.
Thanks for the info. I would guess that at least to begin with Apple would stick to gathering data and avoid any medical claims or sending data to medical professionals. It would be up to me to take that info to my doctor, run additional tests, and get a professional diagnosis. I think there’s a lot Apple can do before they cross the line into ‘regulation hell’, so to speak.
I would expect, but admittedly, I don’t know, that as these data gathering approaches become prevalent, whether the data is communicated directly or by the patient, that FDA will stick their nose in it. It’s important that the data has integrity. Even in commerce, a scale needs to be certified, and sometimes proven it’s been calibrated on a schedule. More so in healthcare. As we add blood glucose meters, pulse oximetry, etc. this only get’s more involved.
Yet this same FDA does so little to protect us from big pharma or little farma: herbs.
Concerning supplements and herbals, the FDA’s hands have been tied by our Congress critters who are in the pay of the supplement and herbals manufacturers.
I very delighted to find this internet site on bing, just what I was searching for as well saved to fav
Thank you for great content. I look forward to the continuation.